MediPharm Labs Australia Signs First International White-Label Supply Deal with New Zealand based Helius Therapeutics
MediPharm Labs Corp. (TSX: LABS) (OTCQX: MEDIF) (FSE: MLZ) (“MediPharm Labs” or the “Company”) a global leader in specialized, research-driven pharmaceutical-quality cannabis extraction, distillation and derivative products, is pleased to announce that its subsidiary, MediPharm Labs Australia Pty Ltd (“MediPharm Labs Australia”), has entered into an agreement to supply pharmaceutical-quality (GMP certified), white-label, cannabis oil products to Helius Therapeutics Limited (“Helius Therapeutics”), a biotechnology company focused on medicinal cannabis production, distribution and research and development.
This agreement represents MediPharm Labs Australia’s first international supply agreement and is to export finished oil products to New Zealand based Helius Therapeutics. Through its established distribution relationships, Helius Therapeutics will distribute the medicinal cannabis products under its own brand to licensed pharmacies across New Zealand. In New Zealand, medicinal cannabis products must not be in a form intended for smoking and require a physician’s script for unapproved medicines.
“We are excited to be working with Helius Therapeutics, true industry leaders in medicinal cannabis production, distribution and clinical research committed to bringing pharma quality cannabis products to patients across New Zealand,” said Warren Everitt, Chief Executive Officer, MediPharm Labs Australia. “New Zealand is poised to see increased demand from patients for medicinal cannabis as new rules put in place this month are expected to make it easier, while providing more confidence, for doctors looking to prescribe medicinal cannabis products. With our expertise, pharma-focused team of scientists, and global supply chain of Good Manufacturing Practice (GMP) oil-based product, we are positioned well to meet demand and the new minimum quality standards set out by regulators that require all medicinal cannabis products distributed in New Zealand be manufactured under GMP.”
Under the agreement, which has an initial two-year term, MediPharm Labs Australia will supply a range of GMP certified cannabis oil products including a “High-CBD”, a “Balanced” and a “High-THC” formulation in a sublingual dose form.
“New Zealand is one of the world’s newest and most dynamic medicinal cannabis markets. Under new regulations enacted this month, Helius Therapeutics is excited to begin serving patients through healthcare professionals and licensed pharmacies across the country,” said Paul Manning, Chief Executive Officer, Helius Therapeutics. “We are thrilled to be partnering with MediPharm Labs Australia, coupling one of New Zealand’s largest medicinal cannabis companies with one of the world’s leading cannabinoid wholesalers. Even more encouraging, is our ability to remain ahead of the curve as we look to source new active pharmaceutical ingredients and innovative products from MediPharm Labs as we expand our offerings to meet evolving patient needs.”
The agreement will become effective upon confirmation from the New Zealand Medicines and Medical Devices Safety Authority (Medsafe) that the products meet applicable New Zealand quality and regulatory standards for medicinal cannabis products.
About Helius Therapeutics
Helius Therapeutics is a biotechnology company focused on medicinal cannabis research and development and distribution. Helius Therapeutics has designed a state-of-the-art facility in Auckland with controlled growing systems, integrated extraction site, advanced cannabinoid research laboratories and manufacturing operations. As New Zealand’s regulatory environment changes, Helius Therapeutics is poised to set the standard for effective and accessible medicinal cannabis products in New Zealand and beyond.
About MediPharm Labs
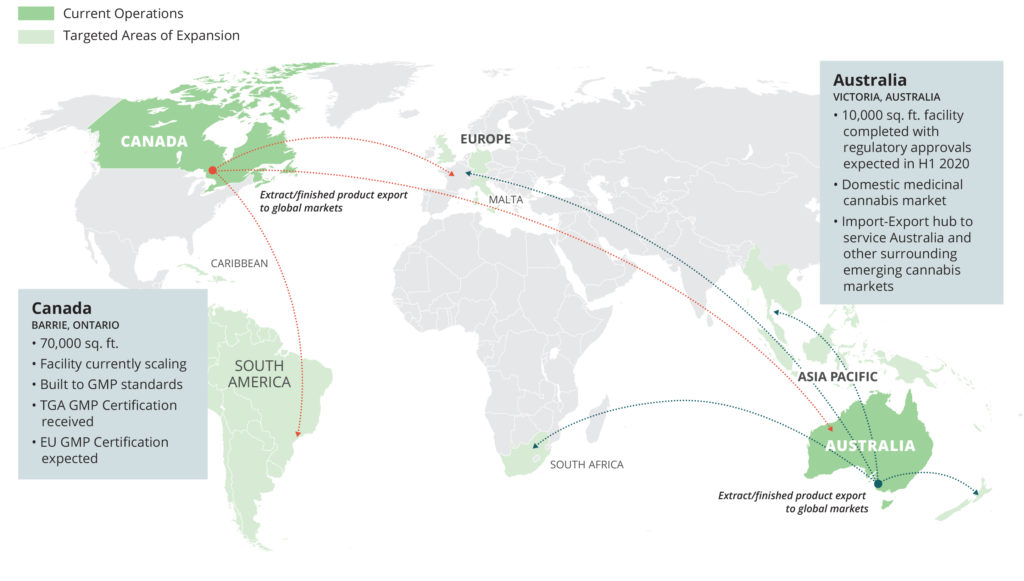
Founded in 2015, MediPharm Labs specializes in the production of purified, pharmaceutical-quality cannabis oil and concentrates and advanced derivative products utilizing a Good Manufacturing Practices certified facility with ISO standard-built clean rooms. MediPharm Labs has invested in an expert, research driven team, state-of-the-art technology, downstream purification methodologies and purpose-built facilities with five primary extraction lines for delivery of pure, trusted and precision-dosed cannabis products for its customers. Through its wholesale and white label platforms, MediPharm Labs formulates, develops (including through sensory testing), processes, packages and distributes cannabis extracts and advanced cannabinoid-based products to domestic and international markets. As a global leader, MediPharm Labs has completed commercial exports to Australia and is nearing commercialization of its Australian extraction facility. MediPharm Labs Australia was established in 2017.